|
|
|||
Electrically Evoked Otoacoustic EmissionsJiefu Zheng, Yuan Zou 1. IntroductionElectrically evoked otoacoustic emissions (EEOAEs) are acoustic signals emitted from the cochlea to the external ear canal when alternating current is delivered into the cochlea (Hubbard and Mountain, 1983; Mountain and Hubbard, 1989; Murata et al., 1991; Ren and Nuttall, 1995). Unlike acoustically evoked OAEs, the EEOAEs are considered ?non-natural? because the cochlea is under the artificial situation stimulated with electrical currents rather than sounds. In addition, due to the need to open the bulla and place stimulation electrodes into/onto the cochlea, the procedures for EEOAE measurement are invasive. In general, there are two ways to apply current injection, one being the intracochlear and the other being the extracochlear stimulation. To our knowledge, the published reports of EEOAE measurements were conducted on animals. Animal species used for EEOAE research include gerbil, guinea pigs, chinchilla, lizards and chicken (Hubbard and Mountain, 1983; Nuttall and Ren, 1995; Sun et al., 2000; Manley 2001; Chen et al., 2001). The EEOAEs can always be obtained in normal ears. 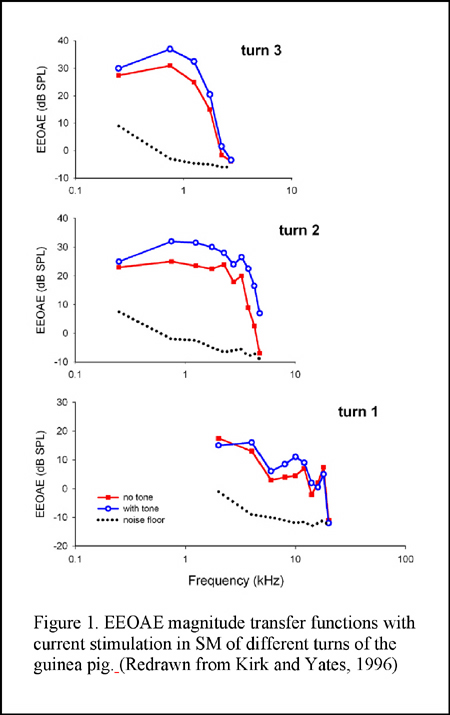 The EEOAEs occur at the same frequency as that of the electrical stimulus as a result of fast electromotile responses of the outer hair cells (OHCs) that underlie the reverse transduction process (i.e., electrical-to mechanical transduction) (Mountain and Hubbard, 1989; Nuttall and Ren, 1995). They are reduced or absent in animals in which OHCs are damaged after treatment with ototoxic drugs or by acoustic injury (Ren and Nuttall, 2000; Reyes et al., 2001; Zheng et al., 2001; Nakajima et al., 1996). Experiments on basilar membrane (BM) motion induced by electrical stimulation indicate that OHCs undergoing electrically evoked motion are capable of producing high-fidelity, high frequency mechanical energy. The electrically evoked intracochlear energy results in conventional traveling waves within the cochlea, as well as emissions of sound from the cochlea (Xue et al., 1995; Nuttall and Ren, 1995). It is a consensus that the EEOAEs are generated at the location near the stimulation electrode. However, the mechanisms of EEOAE generation differ with methods for current delivery. It is thought that electrical currents delivered to the scala media (SM) pass through the transduction channels. Thus the EEOAEs are generated or modulated by mechanisms related to cochlear transduction (Yates and Kirk, 1998). In contrast, current delivered into the scala tympani (ST) (directly or from round window stimulation) may extracellularly stimulate the OHCs by directly affecting the transmembrane voltage of OHCs, resulting in voltage-dependent OHC motion (Ren and Nuttall, 1995; Nuttall and Ren, 1995; Nuttall et al., 2001).
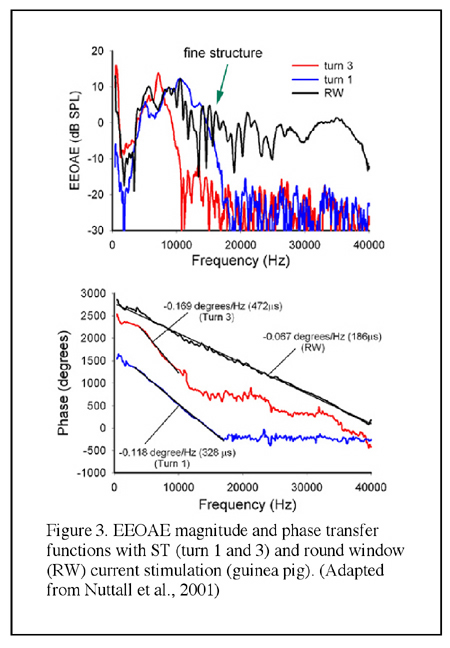
 SM-applied current is thought to stimulate a spatially well-defined population of OHCs due to the confining effect of the SM space constant, which is estimated to be 1.5 to 4 mm (Xue et al., 1993; Nakajima et al., 1994). The space constant is frequency-dependent and as such it could be significantly shorter than 1.5 mm in the cochlear basal turn. The magnitude transfer functions (i.e., the magnitude of emissions as a function of the frequency) are relatively narrow band or low-pass functions and the slope of the phase-versus-frequency function of the emission (the group delay) is related to the tonotopic location of the SM electrode (Murata et al., 1991; Nakajima et al., 1994; Kirk and Yates, 1996) (see Figures 1 and 2). Similarly, currents applied in the ST result in emissions with tonotopically organized band-pass bandwidth properties as well (Nuttall et al., 2001) (Figure 3). In contrast, EEOAE transfer functions from passing current onto the round window (RW) are considerably broader in frequency (being from 60 Hz to 50 kHz in guinea pigs) with a group delay apparently consistent with the expected reverse propagation time from cochlear locations near the round window (Ren and Nuttall, 1995; Nuttall et al., 2001) (Figure 3).
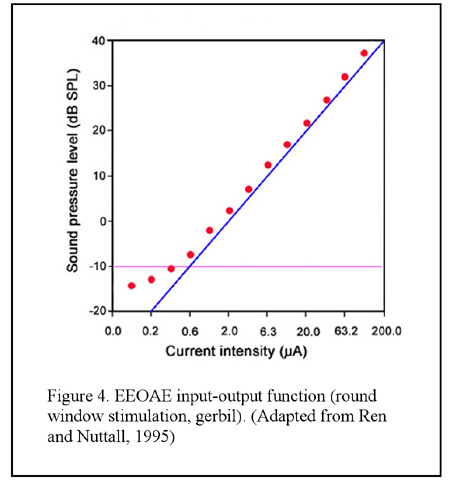
When sweeping the electrical current with small frequency steps the resultant EEOAE transfer function shows peaks and notches in magnitude, the appearance being termed as ?fine structure?, which is similar to that has been observed in the DPOAE gram (Figure 3). Unlike the acoustically evoked OAEs, the EEOAE input-output function is generally linear even in sensitive cochlea (Figure 4), though nonlinearity could be observed in certain conditions (Ren and Nuttall, 1995; Nakajima et al., 1998). Nevertheless, simultaneously applied electrical currents at two frequencies (f1 and f2) are able to evoke DPOAEs as what are evoked by acoustic primaries (Ren et al., 1996) EEOAEs can be modulated by an acoustic stimulus. It was firstly reported by Mountain and Hubbard and then by others that a simultaneously presented sound could enhance the EEOAEs evoked by currents applied into the SM (Mountain and Hubbard, 1989; Xue et al., 1993; Nakajima et al., 1994; Kirk and Yates, 1996) (see Figure 1). The enhancement is largest when the acoustic frequencies are near the characteristic frequency (CF) of the current injection location (Xue, et al., 1993). This enhancement is hypothesized as being the result of the opening of the negative feedback loop of the cochlear amplification by acoustic stimulation that causes reduction of the forward transduction. The ?opening? of the feedback loop, in this hypothesis, removes the suppression of the mechanical response and consequently results in an increased amplitude of the EEOAEs (Mountain and Hubbard, 1989). However, data inconsistent with this hypothesis were reported by others (Kirk and Yates, 1996). In round window evoked EEOAE study, it was observed that a high sound level acoustical tone enhanced the EEOAE fine structure at frequencies below that of the acoustical stimulus, and suppressed the overall level of the EEOAEs at frequencies above the acoustical frequency. The acoustic modulations on the EEOAEs was most efficient for frequencies approximately one half octave lower than the acoustical frequencies (Ren and Nuttall, 1998). The underlying mechanisms for these modulations were hypothesized to be the interaction between the electrically and acoustically evoked basilar membrane motions and/or the acoustical stimulus induced basilar membrane impedance discontinuity at its CF location that leads to a change of the electrically evoked traveling waves (Ren and Nuttall, 1998). 
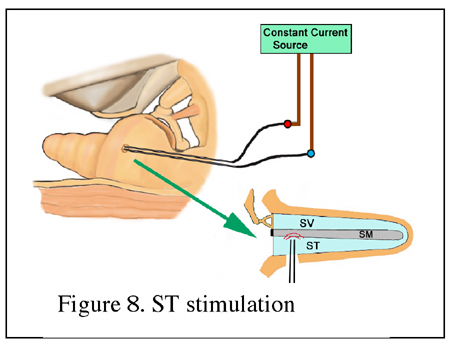
There is increasing evidence in favor of the hypothesis that the EEOAEs recorded in the ear canal have more than one origin on the BM. By using multiple component analysis method, a long delay component (LDC) and a short delay component (SDC) of EEOAEs were identified (Ren and Nuttall, 2000; Zou et al, 2003) (Figure 5). By observing the effects of furosemide, quinine, and other pathophysiological cochlear conditions on the multiple components of EEOAEs (Ren and Nuttall, 1998, 2000; Zheng et al, 2001), it was found that the LDC of the EEOAEs is closely related to the cochlear sensitivity and is vulnerable to cochlear damage. It was also found that the fine structure of EEOAEs was a feature of sensitive cochlea and depended on the presence of the LDC. The fine structure is postulated to result from the cancellation/enhancement effects of multiple sound waves in healthy cochlea. In contrast, the overall magnitude of EEOAEs is mainly related to the SDC and is relatively less sensitive to cochlear damage. Therefore, it is hypothesized thatelectrical currents delivered into the cochlea result in OHC motion and hence the basilar membrane vibration at the site near the stimulation electrode, giving rise to energy propagation to both forward (to apical) and backward (to basal) directions. The energy propagating backwards to the oval window results in stapes vibration, giving rise to the SDC. Whereas, the energy propagating forward will reflect from its own CF location, forming the LDC (Ren and Nuttall, 2000; Zou et al, 2003) (Figure 6).  2. Methods of EEOAE measurementExperimental animals need to be anesthetized. A microphone is coupled to the ear canal to measure the acoustic signal generated in the cochlea by the electrical stimulation. In order to expose the cochlea for placement of the stimulation electrodes the bulla needs to be opened. The middle ear muscle tendons should be sectioned to avoid electrically evoked muscle contractions. The stimuli are usually sinusoidal constant currents delivered through an optically-isolated constant-current source. The acoustic signals in the ear canal are recorded in terms of magnitude and phase at the frequency of the electrical current. Two approaches for electrical current stimulation are used to evoke the OAEs: (1) Intracochlear stimulation. Holes are made in the cochlea and electrodes are placed into the cochlea to deliver the current stimuli. According to the location of the electrode placement, there are two types of intracochlear stimulation, the scala media stimulation and scala tympani stimulation. (2) Extracochlear stimulation. The electrode is placed in the round window niche so that the current is delivered through the round window membrane into the scala tympani. The cochlea is kept intact in this situation. Scala media stimulation: A glass microelectrode is inserted into the scala media through the lateral wall or the basilar membrane (Figure 7). Constant current is injected into the scala media using the microelectrode. The tip diameter of the electrode is approximately 5 mm and the microelectrode is filled with 0.16 M, 1 M, or 3 M KCl. A Ag/AgCl wire is inserted into the neck muscles to serve as the current return electrode. The intensity of the currents could range from 1 to 50 mA peak-to-peak.
3. Data analysis and presentation ? Magnitude and phase transfer functionsMagnitude and phase are essential information for EEOAEs research. The magnitude transfer function curve is obtained by plotting the magnitude as a function of the frequency, which provides information of the frequency response or bandwidth features (Figure 1 and Figure 3). As that has been mentioned in the Introduction, the EEOAE magnitude transfer function presents a feature of ?fine structure? in sensitive cochlea. While the overall level of the EEOAEs rises as the current intensity increase, the amplitude of fine structure (the peaks and notches in magnitude transfer function) tends to decrease with the increasing mean EEOAE level. When the phase (in degree or radian) is plotted against the frequency the phase transfer function curve will be obtained in that group delay could be calculated from the slope (see Figure 2 and Figure 3).
? Input-output function By plotting the magnitude of EEOAEs as a function of the electrical current intensity the input-output function (I/O function) is obtained. The I/O function of the EEOAEs is generally linear (see Figure 4). ? Multiple component analysis The presence of the fine structure suggests the existence of multiple sources of the EEOAEs and consequently, multiple components of the recorded emissions. A method of multiple component analysis (MCA) has been developed (Ren and Nuttall, 2000) in that the real part of the emission is calculated from the magnitude and phase spectra and then the multiple components are extracted. By using the MCA for EEOAE data analysis, long delay component (LDC) and short delay component (SDC) are observed (Figure 5 and Figure 10).  4. Hearing sensitivity and EEOAEsIt has been demonstrated that the OHCs are the origin of the EEOAEs. Thus the EEOAEs provide a tool for the study of in vivo OHC electromotility. The magnitude and the fine structure (in the magnitude-frequency transfer function) of the EEOAEs are associated with the cochlear sensitivity. Any kind of insult to the cochlea that inhibits the OHC function will result in EEOAEs fine structure diminution and/or magnitude reduction. The fine structure is a feature of the sensitive cochlea and is very vulnerable to any damage on the OHCs whereas the overall mean magnitude of EEOAEs is relatively less sensitive to the damage. In fact, the reduction of the EEOAE magnitude is not proportional to the cochlear sensitivity loss. Even in the case when the animal is dead and the fine structure is abolished, the overall magnitude of EEOAEs is reduced by only about 15-20 dB, and residual EEOAEs still can be observed. Moreover, total loss of hair cells further reduces the EEOAE magnitude but generally residual emissions are still seen.
References: Chen L, Sun W, Salvi RJ., Electrically evoked otoacoustic emissions from the chicken ear. Hear Res. 2001 Nov;161(1-2):54-64. Hubbard, A.E., Mountain, D.C., 1983. Alternating current delivered into the scala media alters sound pressure at the eardrum. Science, 222, 510-512. Kirk, D.L., Yates, G.K., 1996. Frequency tuning and acoustic enhancement of electrically evoked otoacoustic emissions in the guinea pig cochlea. J. Acoust. Soc. Am. 100, 3714-3725. Mountain, D.C., Hubbard, A.E., 1989. Rapid force production in the cochlea. Hear. Res. 42, 195-202. Manley, GA., 2001. Evidence for an active process and a cochlear amplifier in nonmammals. J. Neurophysiol. 86(2), 541-9. (Review) Murata, K., Moriyama, T., Hosokawa, Y., Minami, S., 1991. Alternating current induced otoacoustic emissions in the guinea pig. Hear. Res. 55, 201-214. Nakajima, H.H. and Olson, E.S., 1994. Electrically evoked otoacoustic emissions from the apical turns of the gerbil cochlea. J.Acoust. Soc. Am. 96 (2), 786-794. Nakajima, H.H., Olson, E.S., Mountain, D.C., and Bubbard A.E., 1996. Acoustic overstimulation enhances low-frequency electrically-evoked otoacoustic emissions and reduces high-frequency emissions. Auditory Neuroscience. 3, 79-99. Nuttall, A.L., Ren, T., 1995. Electromotile hearing: evidence from basilar membrane motion and otoacoustic emissions. Hear. Res. 92, 170-177. Nuttall, A.L., Zheng, J., Ren, T., de Bore, E., 2001. Electrically evoked otoacoustic emissions from apical and basal perilymphatic electrode positions in the guinea pig cochlea. Hear. Res. 152, 77-89. Ren, T., Nuttall, A.L., 1995. Extracochlear electrically evoked otoacoustic emissions: a model for in vivo assessment of outer hair cell electromotility. Hear. Res. 92, 178-183. Ren, T., Nuttall, A.L., Miller, J.M., 1996. Electrically evoked cubic distortion product otoacoustic emissions from gerbil cochlea. Hear. Res. 102 (1-2), 43-50. Ren, T., Nuttall, A.L., 1998. Acoustic modulation of electrically evoked otoacoustic emission in intact gerbil cochlea. Hear. Res. 120, 7-16. Ren, T., Nuttall, A.L., 2000. Fine structure and multicomponents of the electrically evoked otoacoustic emission in gerbil. Hear. Res. 143, 58-68. Reyes S, Ding D, Sun W, Salvi R., 2001. Effect of inner and outer hair cell lesions on electrically evoked otoacoustic emissions. Hear Res. 158, 139-50. Sun, W., Ding, D., Reyes. S., Salvi R.J., 2000. Effects of AC and DC stimulation on chinchilla SOAE amplitude and frequency. Hear Res. 150, 137-148. Xue, S., Mountain, D.C., Hubbard, A.E., 1993. Acoustic enhancement of electrically-evoked otoacoustic emissions reflects basilar membrane tuning: Experiment results. Hear. Res. 70, 121-126. Xue, S., Mountain, D.C., Hubbard, A.E., 1995. Electrically evoked basilar membrane motion. J. Acoust. Soc. Am. 97, 3030-3041. Yates G.K. and Kirk D.L., 1998. Cochlear electrically evoked emissions modulated by mechanical transduction channels. J. Neurosci. 18 (6), 1996-2003. Zheng, J., Ren, T., Parthasarathi, A., Nuttall, A.L., 2001. Quinine induced alterations of electrically evoked otoacoustic emissions and cochlear potentials in guinea pigs. Hear. Res. 154, 124-134. Zou Y. Zheng, J., Nuttall, A.L., Ren, T., 2003. The sources of electrically evoked otoacoustic emissions, Hear. Res. 180, 91-100
|